Important Notice
How does the EndoStim System work?
GERD and associated symptoms are often caused by a dysfunctional lower esophageal sphincter (LES) valve between the stomach and the esophagus. The EndoStim neurostimulation system delivers mild electrical signals to the LES automatically throughout the day. This gentle stimulation is designed to restore normal function to the LES, and normally cannot be felt by patients.
The EndoStim System gently stimulates the LES valve to allow it to function normally again – stay closed to prevent reflux, open to allow for food and drink to pass into the stomach.
The EndoStim System gently stimulates the LES valve to allow it to function normally again – stay closed to prevent reflux, open to allow for food and drink to pass into the stomach.
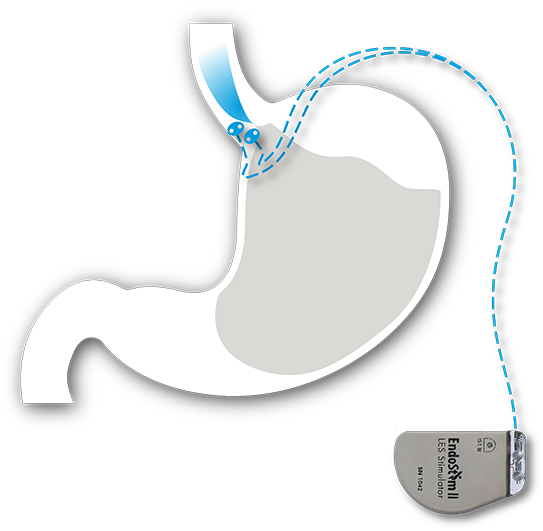
The EndoStim System
The EndoStim System consists of an implantable neurostimulation device and lead placed via a quick, minimally-invasive laparoscopic procedure. The system is designed to provide long-term reflux control by automatically delivering mild electrical signals throughout the day to the patient’s weak or dysfunctional lower esophageal sphincter (LES) muscle (the underlying cause of GERD).
Delivering EndoStim Therapy
The EndoStim System is placed through a minimally-invasive procedure. Unlike traditional anti-reflux surgery, the EndoStim System is designed to preserve the body’s natural anatomy in order to reduce or avoid gastro-intestinal side effects.
- Minimally-invasive procedure of about 1 hour
- 2 small electrodes placed on the lower esophagus
- EndoStim neurostimulator placed under the skin (in the abdomen)
- Quick recovery
Once placed, the EndoStim System delivers stimulation therapy to the LES via the lead. the EndoStim System is programmed wirelessly by a physician using an external handheld controller. The System automatically stimulates the LES to allow it to function normally without any sensation to the patient.
The stimulation therapy can, if needed, be adjusted at future follow-up visits by a physician, and the device battery is expected to last approximately six years under recommended stimulation algorithms.
The EndoStim System has been successfully investigated in multiple independent international studies.
- Minimally-invasive procedure of about 1 hour
- 2 small electrodes placed on the lower esophagus
- EndoStim neurostimulator placed under the skin (in the abdomen)
- Quick recovery
Once placed, the EndoStim System delivers stimulation therapy to the LES via the lead. the EndoStim System is programmed wirelessly by a physician using an external handheld controller. The System automatically stimulates the LES to allow it to function normally without any sensation to the patient.
The stimulation therapy can, if needed, be adjusted at future follow-up visits by a physician, and the device battery is expected to last approximately six years under recommended stimulation algorithms.
The EndoStim System has been successfully investigated in multiple independent international studies.
